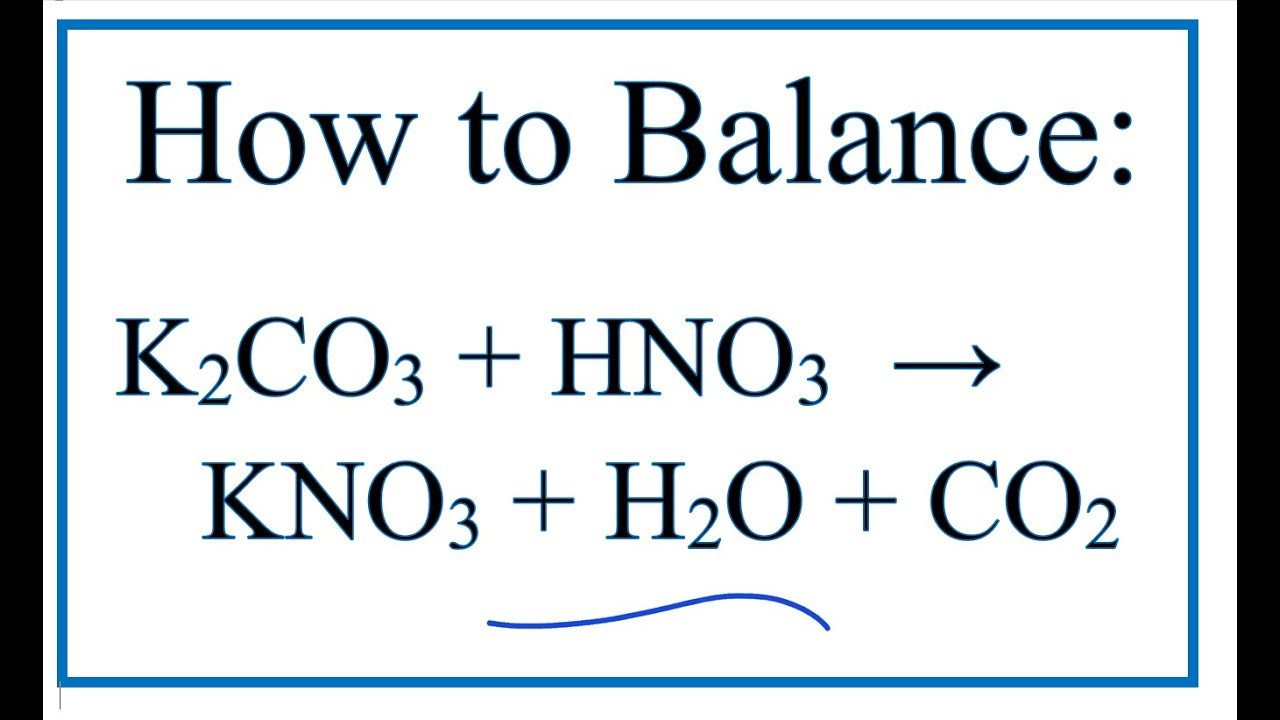
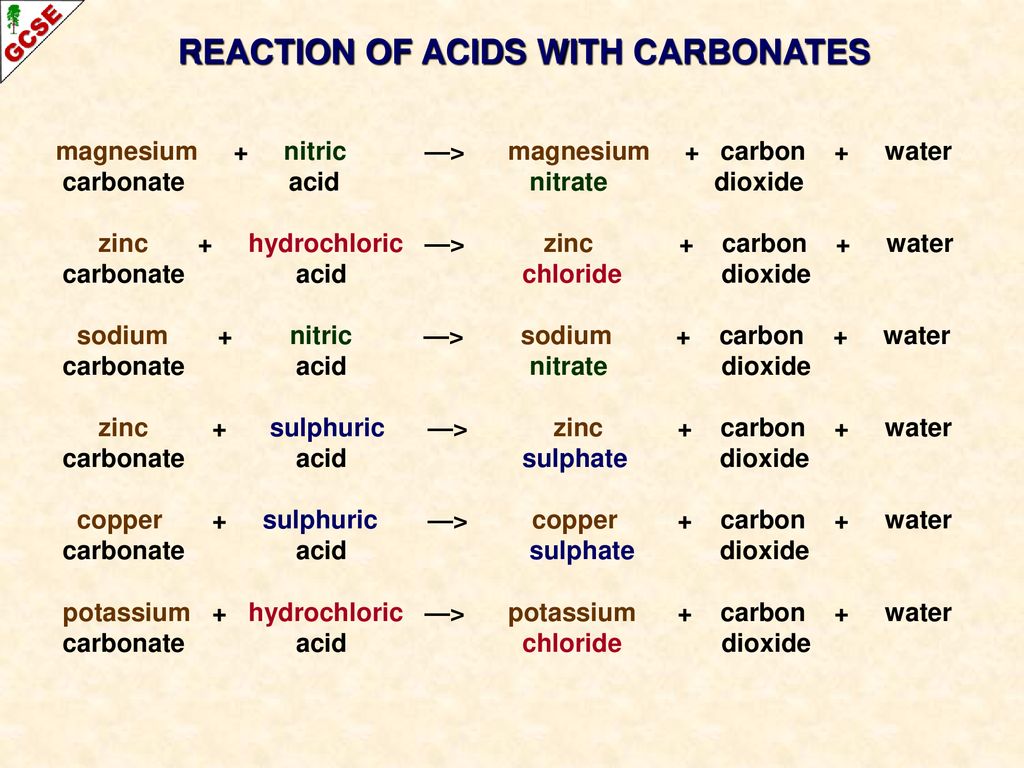
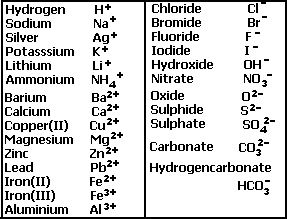
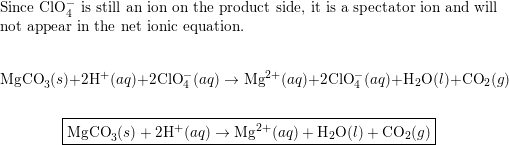OneClass: 1a)When magnesium carbonate, MgCO3, is treated with a dilutesolution of nitric acid, HNO3, ...
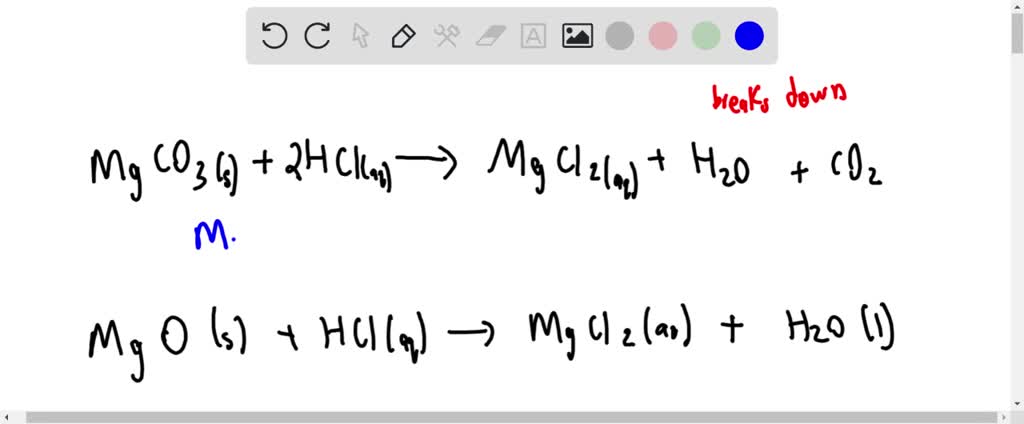
SOLVED: JM Question 9 (10 marks = 6 marks + 4 marks) In Lab 3, chemical reaction was performed by the addition of solid sodium hydrogencarbonate to a small volume of 2
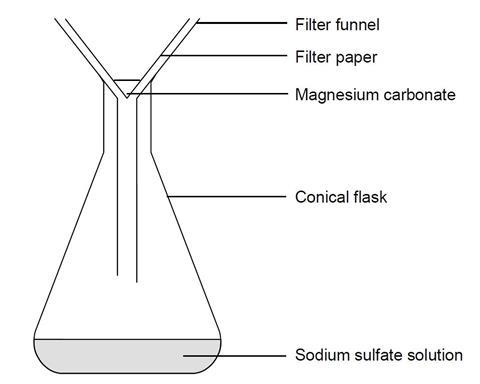
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education

What are Acids? An acid is any compound that yields hydrogen ions (H + ) or hydronium ions (H 3 O + ) when dissolved in water. Hydronium ions are really. - ppt download
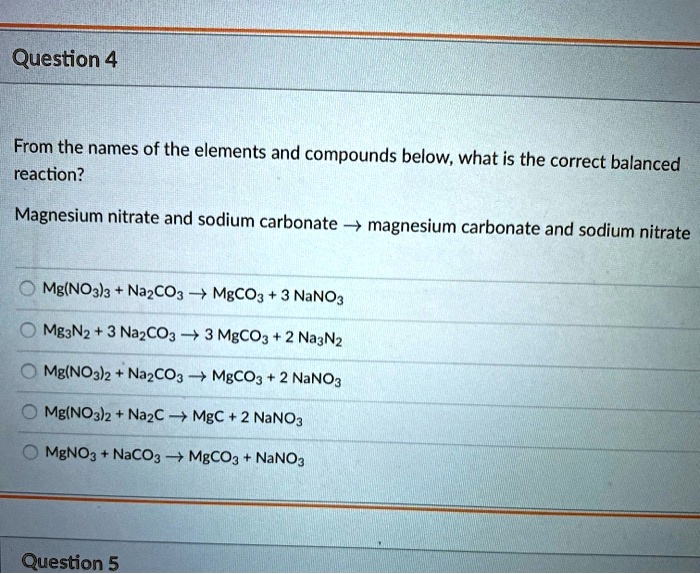
SOLVED: Question 4 From the names of the elements and compounds below, what is the correct reaction? balanced Magnesium nitrate and sodium carbonate magnesium carbonate and sodium nitrate Mg(NO3l3 NazCO3 MgCOz NaNO3

EP0858985A2 - Aqueous alkaline earth nitrate fertilizer composition and process for making same - Google Patents