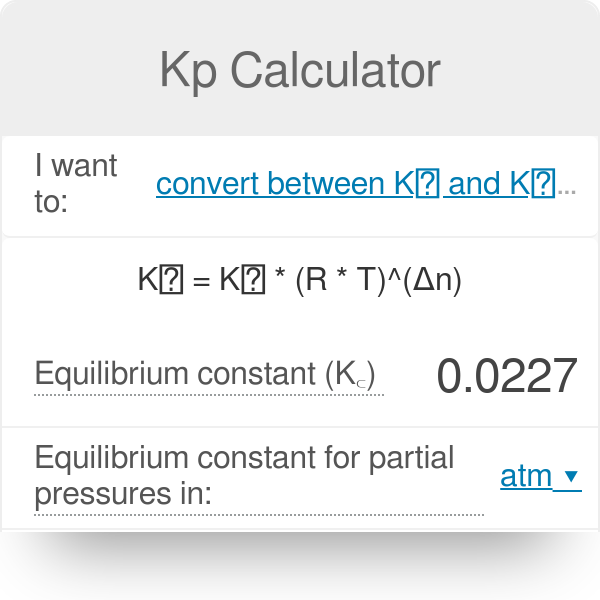
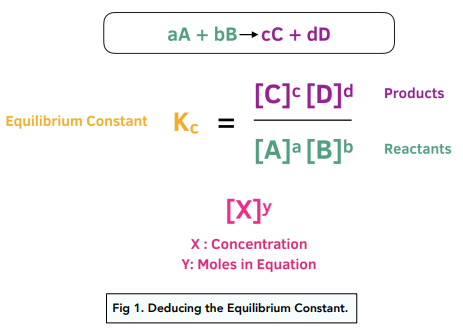
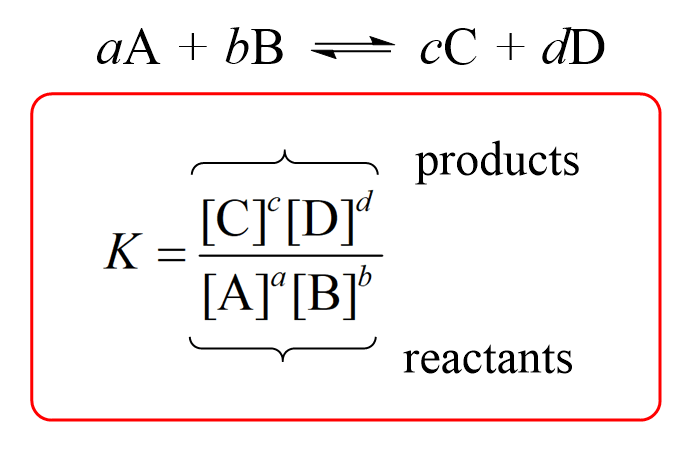
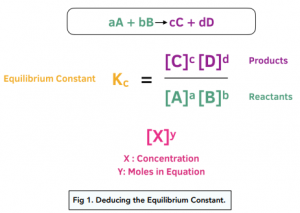
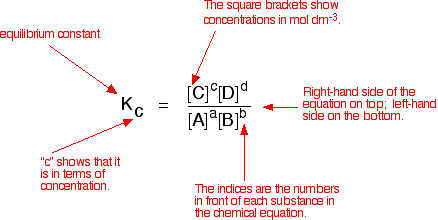
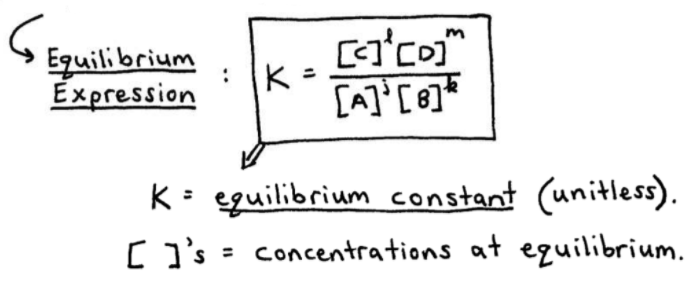
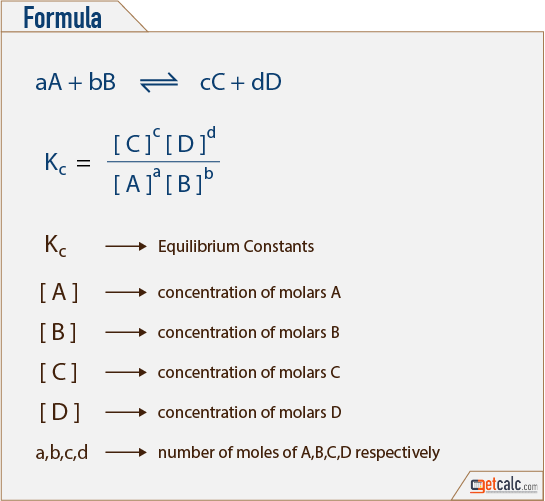
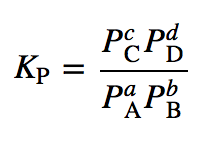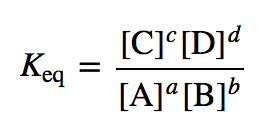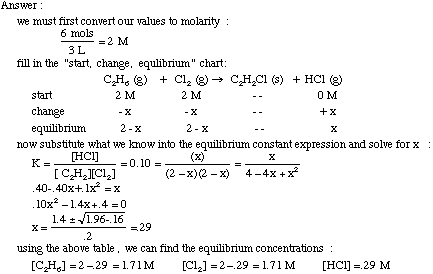Equilibrium constant has a unit when the number of moles on both sides are not equal, why? If number of moles are not equal then there is no equilibrium then how equilibrium

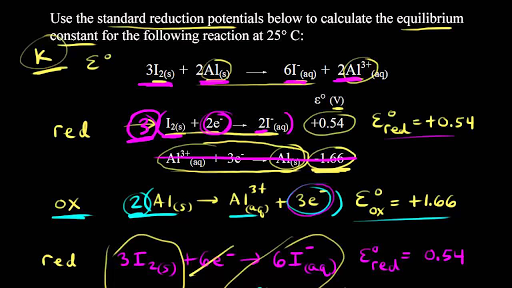
Calculate the equilibrium constant the reaction, 25^oCCu(s) + 2Ag^+ (aq) → Cu^{+2} (aq) + 2Ag (s)at 25^oC, E^ocell = 0.47 V, R = 8.134 JK^{-1} F = 96500 C is

SOLVED: Calculate the equilibrium constant for the following reaction at 25°C: Zn2+(aq) + 2Ag(s) ⇌ Zn(s) + 2Ag+(aq) Round your answer to one significant figure. Enter your answer in scientific notation. Note,

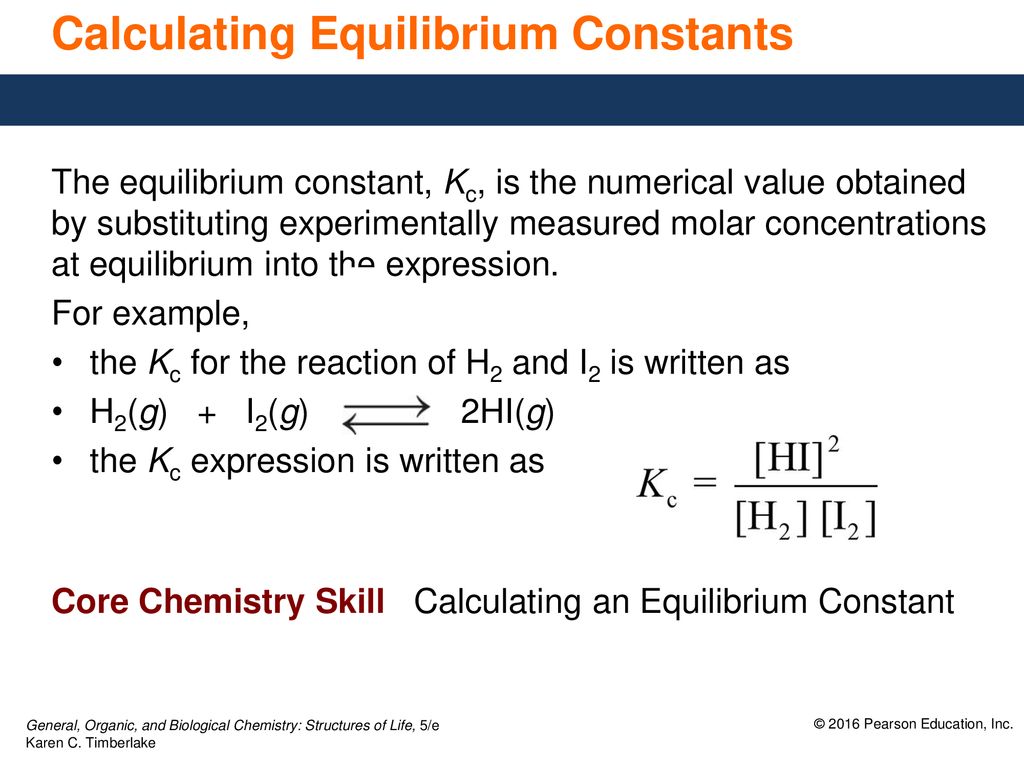
Calculating equilibrium constants from equilibrium concentrations or partial pressures (worked examples) (video) | Khan Academy

Learn how to calculate an equilibrium constant Kc. | Chemistry lessons, Teaching chemistry, Chemistry education
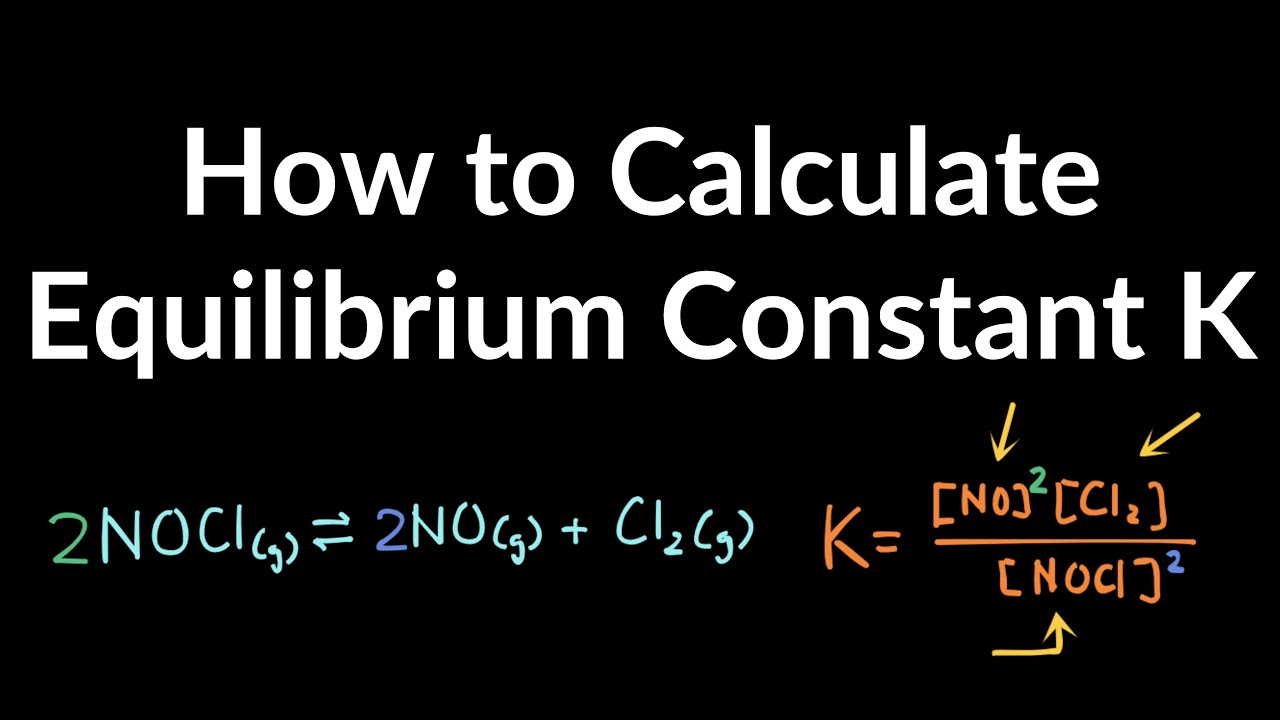
How to Calculate Equilibrium Constant K Value Practice Problems & Exampled Explained Step by Step - YouTube