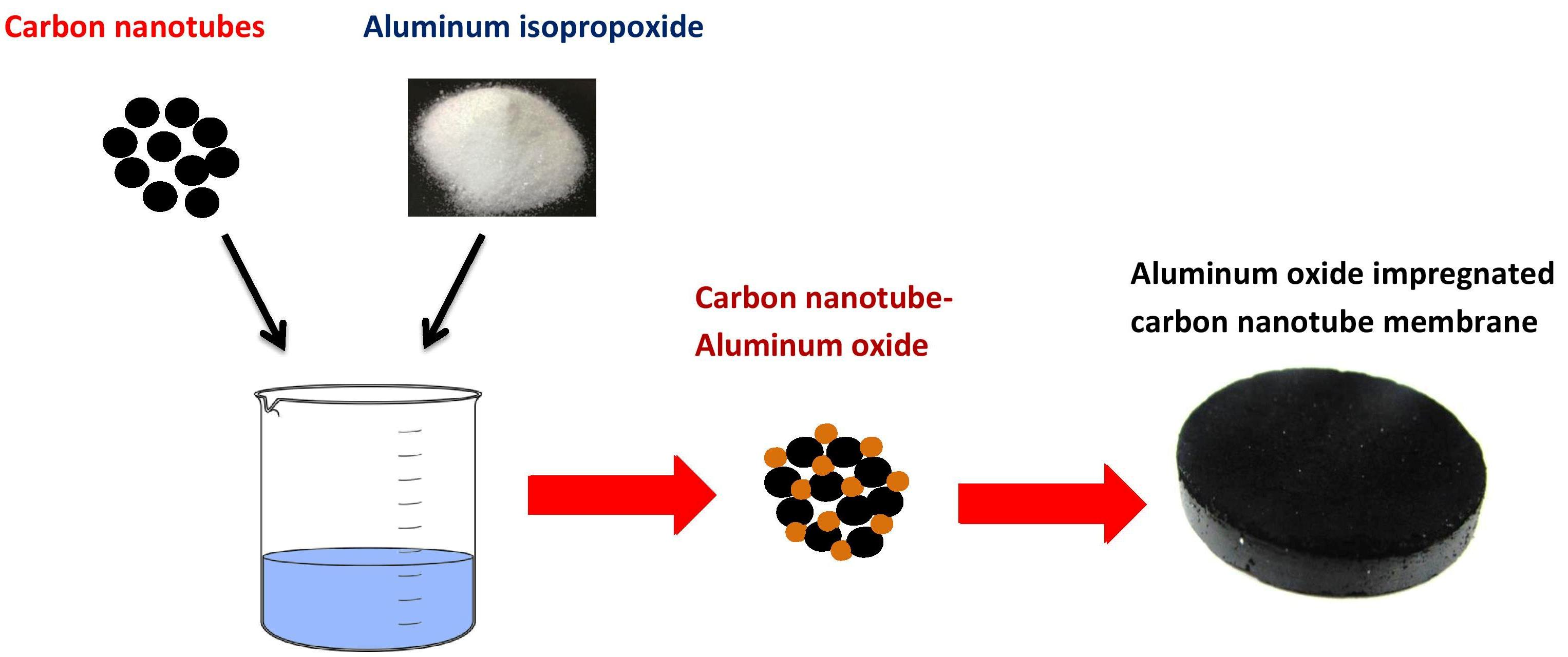
Materials | Free Full-Text | Novel Aluminum Oxide-Impregnated Carbon Nanotube Membrane for the Removal of Cadmium from Aqueous Solution

a) Carbon concentrations in the bulk of a 100 nm-thick aluminum oxide... | Download Scientific Diagram
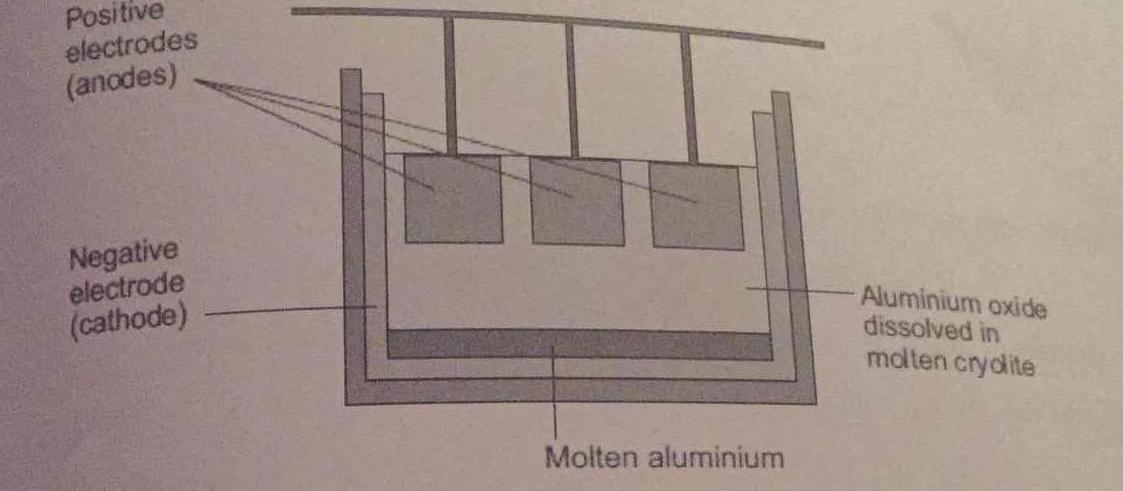
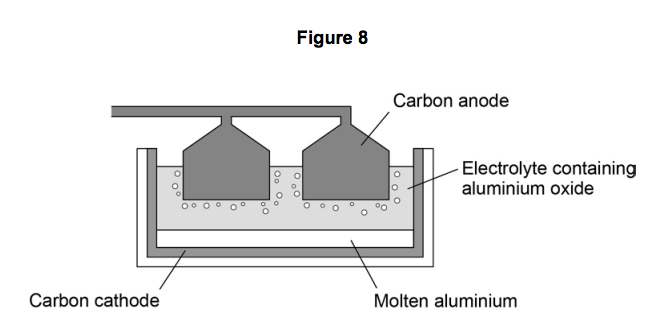
Aluminium is extracted by electrolysis, as shown in the figure. Why can aluminium not be extracted by heating aluminium oxide with carbon? | Socratic

Raman spectra of CNTs before and after the ALD Aluminium Oxide coating | Download Scientific Diagram

Aluminium oxide contains 52.9% aluminium and carbon dioxide contains 27.27% carbon. Assuming the....

scheme of the preparation of the carbon-coated anodic aluminum oxide... | Download Scientific Diagram

in a electrolytic tank aluminium metal is being extracted by d electrolysis of molten aluminium oxide using carbon electrodes.Ot is observed that one of the carbon electrodes is gradually burnt away and

Electrophoretic deposition of multi-walled carbon nanotubes on porous anodic aluminum oxide using ionic liquid as a dispersing agent - ScienceDirect
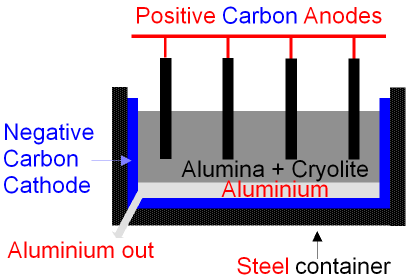
GCSE CHEMISTRY - Extraction of Aluminium - Electrolysis - Ionic Equations - Anode Replacement - GCSE SCIENCE.
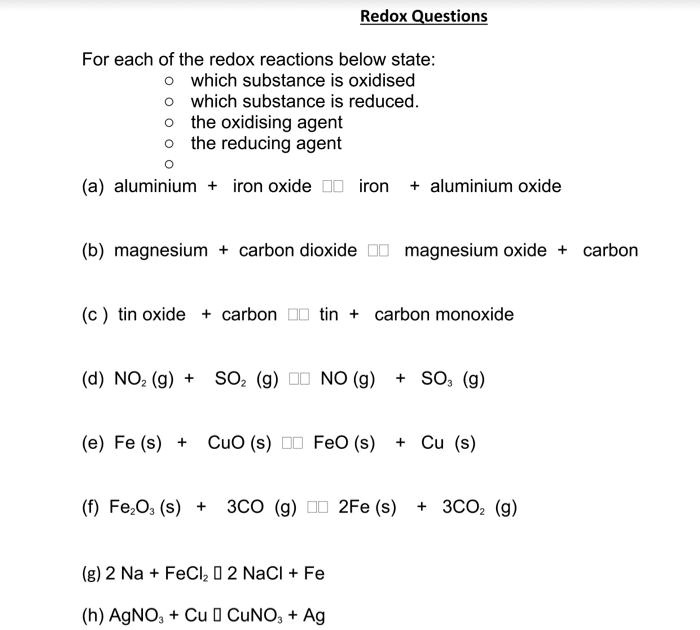
SOLVED: For each of the redox reactions below, state which substance is oxidized and which substance is reduced, as well as the oxidizing agent and the reducing agent. (a) aluminium + iron







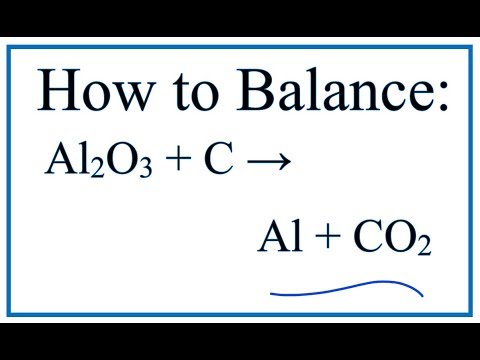
.png)